Are you a rodent? If not, BCAA supplementation may not help you increase muscle mass. Please, let me explain.
Protein and BCAA Basics
Protein is part of every cell in the body. In addition to making enzymes, hormones, and other body chemicals, protein is an important building block of bones, muscles, cartilage, skin, and blood. Protein is made up of 20 amino acids, 9 of which can’t be produced by the body in physiologically sufficient amounts. These 9 amino acids are called essential amino acids (EAAs) and must be supplied by the diet. The other 11 amino acids are called nonessential amino acids (NEAAs); the body can manufacture NEAAs in sufficient amounts and, thus, NEAAs need not be a dietary focus. Muscle protein synthesis, or building muscle protein, has the same amino acid requirements as building any other protein in the body. Since the body can manufacture NEAAs on its own, the ingestion of NEAAs is generally a non-issue for promoting muscle protein synthesis; the limiting factor for muscle protein creation is consuming the EAAs in sufficient quantities [1-4].
Note: You may have noticed that a few words are highlighted in blue; abbreviations for these words are used extensively in this article.
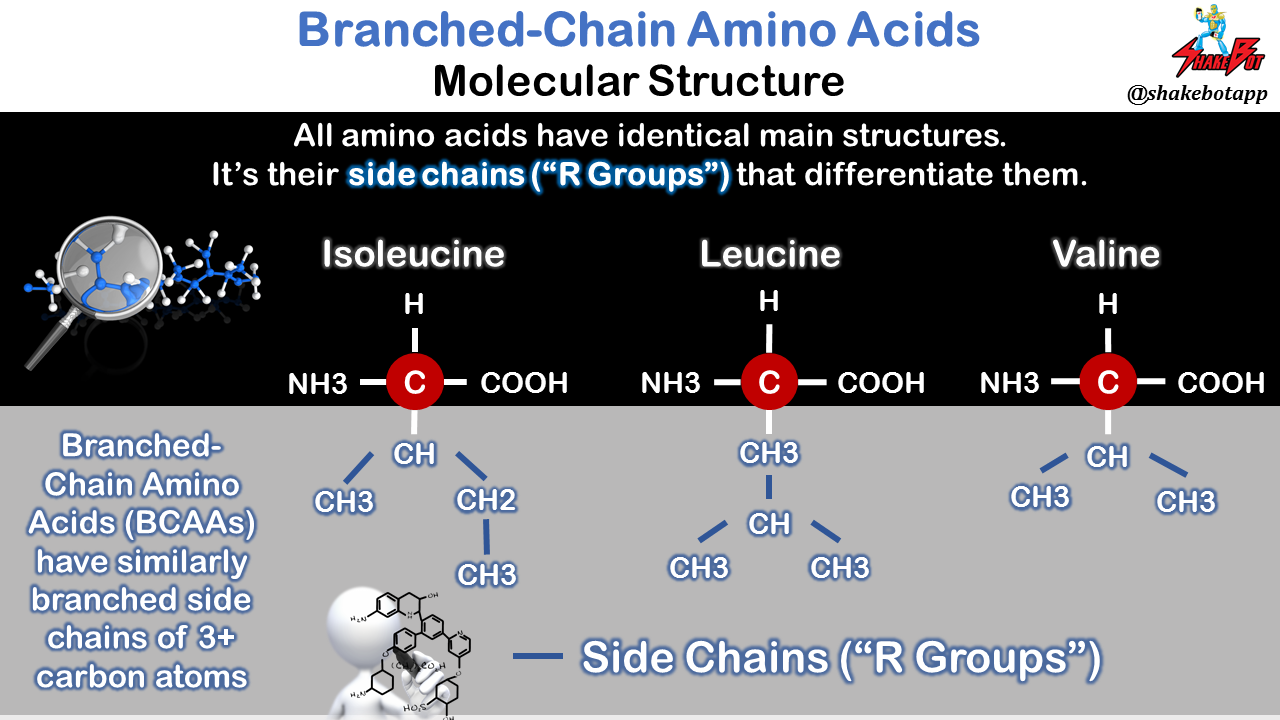
Because isoleucine, leucine, and valine possess a branched side chain in their molecular structure, these three amino acids are called branched-chain amino acids (BCAAs); BCAAs are, also, 3 of the 9 EAAs [5]. The three BCAAs, collectively, make up 35–40% of the total body amino acid pool. BCAAs are plentiful inside muscle tissue [6-9] and are also preferentially metabolized there, whereas the other EAAs are mostly metabolized in the gut or liver [10-12]. Over the past 3.5 decades, there has been a plethora of data put forward suggesting that BCAAs have a unique ability to stimulate muscle protein synthesis (MPS), [13]. More recently, it has been reported that leucine, one of the three BCAAs, also makes a unique contribution towards MPS stimulation, primarily due to its special role in regulating intracellular signaling pathways [14-16]. Let’s take a deeper look at these claims.
Animals vs. Humans and Leucine
Much of the research demonstrating an ergogenic impact of BCAAs on MPS has been performed in rodents [17-20], using procedures and relative infusion quantities that have little applicability in humans. Additionally, there are clear differences in physiology and signaling pathways between rats and humans, which limits the applicability of rodent-driven results [21]. Although relevant, the discussion on BCAA and, specifically, leucine ergogenicity will focus on human studies.
When sub-optimal amounts of protein are consumed, adding leucine can help to optimize MPS [22], particularly in individuals with increased anabolic resistance, like older individuals [23-27]. In a recent study by Atherton et al. (2017), 4.2g leucine added to a sub-optimal protein dose immediately following resistance exercise enhanced MPS in men, both young and old [28]. However, when EAAs are administered in excessive quantities, adding extra leucine to the mix may not provide additional benefit [29].
In a few older experiments by Louard et al., 10 healthy subjects were intravenously infused with BCAAs for 3 hours [30]. When MPS and muscle protein breakdown (MPB) were both reduced, the net muscle protein balance remained negative, resulting in a catabolic state. Five years later, the same research group conducted a similar experiment, but this time infused the subjects with BCAAs for 16 hours [31]. The findings in this experiment were like those of the first; MPS, MPB, and ultimately, muscle protein turnover was reduced, allowing catabolism to persist. Ironically, the infusion of BCAAs in these experiments resulted in MPS decreases, not increases, equating to an overall catabolic effect [30, 31]. In neither of these studies was there a shift from the catabolic to anabolic state. A similar study was done by Nair et al., but with one major difference; leucine was infused, as opposed to all three BCAAs [32]. When leucine was administered alone, there was no change in MPS but there was a significant reduction in MPB, equating to an overall anabolic effect [32].
The Rate Limiting Amino Acid
The concept of the “rate limiting amino acid” is important to understand prior to going any further. When your diet is deficient in even a single EAA, the other amino acids that exist in excess will be degraded for other purposes (not muscle-building). You need all EAAs for them to be useful for muscle protein synthesis [33]. The significance of the “limiting amino acid” for promoting MPS cannot be overstated; scores developed by researchers to estimate protein quality [34, 35] and amino acid requirements in humans [36] are based on the concept of the “limiting amino acid.” As discussed in the aforementioned studies, providing the body with such a large disproportion of amino acids (i.e. the infusion of massive quantities of BCAAs into the body) results in a disproportionate amino acid “pool” in the body and the synthesis of new protein is limited, not by the BCAAs, but by the most deficient EAA in the “pool” at that time. You can ingest countless amounts of BCAAs, but if the body is deficient in even a single EAA, MPS will be limited.
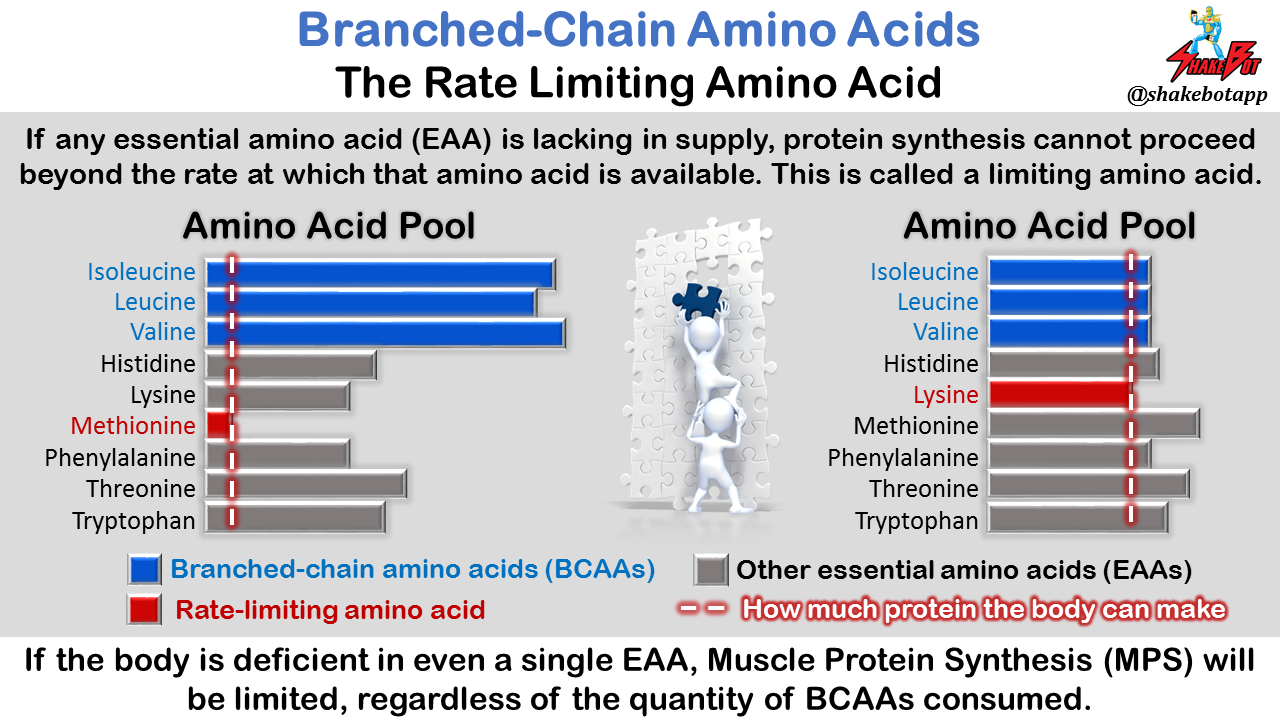
Let’s say you have a box of puzzle pieces. Your goal is to make as many puzzles as possible. If you have hundreds of end pieces, but only a few middle pieces, you will be limited in how many puzzles you can complete by the small number of middle pieces you have available. No matter how many end pieces you have, you cannot complete additional puzzles without middle pieces. How does this apply to BCAAs and protein synthesis? If you supply the body with a plethora of BCAAs, but you have a limited quantity of the other EAAs, muscle protein synthesis (MPS) will be limited by the lack of the other EAAs. A disproportionately large intake of BCAAs without the remaining EAAs necessary to make a complete protein limits the body’s ability to build muscle (and thus, hampers muscle protein synthesis).
Human Physiology and BCAA Ergogenicity for Muscle Growth Don’t Mix
Muscle protein is in a constant state of turnover. In other words, new protein is continuously being synthesized while older proteins are being broken down. Muscle growth/gain occurs when the body is in an anabolic state (i.e. the rate of protein synthesis exceeds the rate of protein breakdown). Muscle losses occur when the opposite occurs and the body is in a catabolic state (i.e. the rate of muscle protein breakdown exceeds the rate of protein synthesis). The primary purpose of BCAA ingestion, or any protein ingestion for that matter, is to maximize the anabolic state of the body [13]. If BCAAs promoted the anabolic state, this would only be possible if there were sufficient quantities of the other EAAs in order for the body to synthesize new protein [3]. Following a meal (post-prandial state) containing a complete protein source, all EAAs are provided in sufficient quantities for the body to utilize, immediately. On the flip side, when hours have gone by without protein intake (post-absorptive state), muscle chips in to help support the energy demands of the body, and only a limited quantity of these released EAAs are available from the muscle tissue breakdown [37].
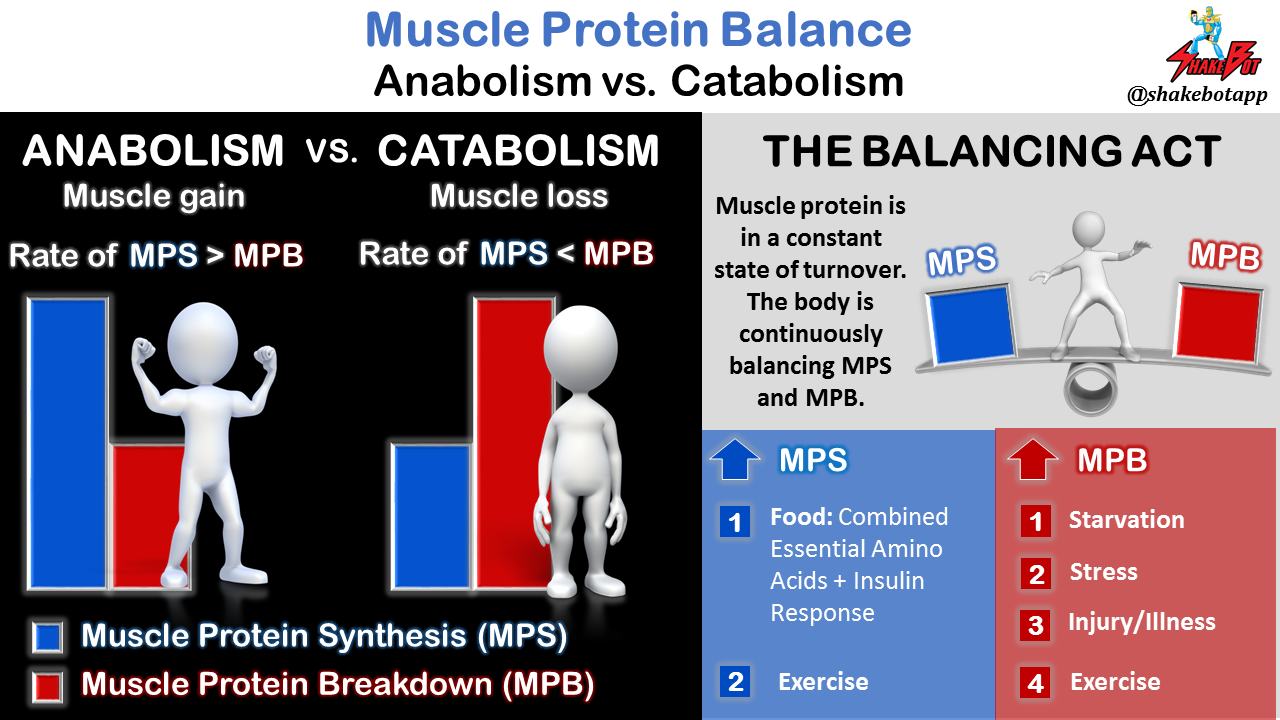
Some of the EAAs released from muscle protein breakdown are partially oxidized within muscle, rendering them unavailable for new muscle protein synthesis. In respect to the BCAA discussion, the EAAs released from muscle that become partially oxidized within muscle can’t be used with the ingested BCAAs to create new protein. The EAAs that are released from muscle tissue that ultimately end up in the free amino acid pool can be used with ingested BCAAs for synthesis of new proteins [38]. It should be noted that muscle protein breakdown (MPB) increases to an even larger degree following exercise, particularly resistance exercise, [2, 46, 47] and this increase is generally more pronounced in untrained individuals [2, 48].
A Little Bit of Research in Humans
In a recent study by Jackman et al. (2017), 11 healthy, resistance-trained young men performed muscle-damaging exercise followed by 5.6g BCAA ingestion [39]. Ingesting BCAAs following exercise increased MPS by 22%, compared with placebo. This stimulation of MPS is far from maximal, which the authors theorize was due to lack of sufficient EAA alongside the BCAA [39]. It’s impossible to be in an anabolic state, even with BCAA ingestion, when the other EAAs (necessary for MPS) are derived from muscle breakdown. An anabolic state cannot occur in the absence of exogenous amino acid intake [13]. BCAAs are thought to stimulate MPS because it they have been reported to stimulate anabolic signaling cascades involved in MPS [11, 50]. However, the measurement of actual MPS was absent from the study design and, as discussed previously, MPS takes place only when there are ample quantities of all required EAAs [14]. In fact, a low dose (3 grams) of EAAs can stimulate MPS without affecting components of the anabolic signaling cascade [40]. Additionally, the anabolic signaling cascade can be stimulated without coinciding MPS. For example, glucose ingestion can stimulate insulin, which increases anabolic signaling without any coinciding increase in MPS (due to EAA deficiency) [41]. Given the aforementioned research and our knowledge of nutritional biochemistry concepts, it’s not farfetched to presume that MPS is limited by availability of all of the EAAs as opposed to anabolic signaling factor activity.
BCAAs Combined with Other Nutrients
When added to a carbohydrate drink, high concentrations of BCAAs in an incomplete amino acid solution showed no benefit for MPS [42]. However, when consumed with a complete protein source (i.e. whey, in this study), additional BCAAs increased MPS [43]. These results likely indicate that one or more of the BCAAs were the rate-limiting amino acid(s) for the stimulation of muscle protein synthesis from whey protein ingestion. In light of what we know, these results make sense; sufficient EAAs are provided via whey protein, which allows for increased MPS, whereas carbohydrate may promote anabolic signaling factors but does not provide sufficient quantities of all EAAs. Other than the unlikely scenario where isoleucine or valine is the rate-limiting amino acid for MPS, there’s no advantage to consuming the three BCAAs collectively over leucine alone. In fact, it appears that the beneficial effects on muscle protein turnover observed with BCAA supplementation is due to the leucine content [43-45].
Potential BCAA Ergogenicity for Exercise Recovery?
Although BCAAs don’t appear to positively impact muscle anabolism, there’s very limited evidence that BCAA consumption can promote recovery through reduced muscle damage and soreness, and faster recuperation of force production capabilities following exercise [49, 51-55]. For example, Jackman et al. (2010) observed attenuated muscle soreness, but not muscle function, when untrained males consumed ~14g BCAA/day in the 3 days following muscle-damaging exercise [51]. Howatson et al. (2012) reported reductions in muscle soreness, damage (i.e. creatine kinase), and faster recuperation of muscle function following muscle-damaging exercise when resistance-trained males consumed 20g BCAA/day for the 7 days prior and 5 days following the exercise bout (12 days of supplementation, total), [52]. A recent systematic review suggests that there may be potential benefits when an array of supplementation criteria is met [49]:
- Frequency: at least 2x/day doses
- Quantity: at least 200 mg/kg/day of BCAA (14 g/day for a 70kg individual)
- Duration: >10 days
- Degree of Damage: low-to-moderate degree of muscle damage
Although there’s a growing body of literature supporting the ability of BCAAs to mitigate outcomes surrounding muscle damage, the jury is still out. In a recent position statement by the International Society of Sports Nutrition, the authors determined that more research is needed to fully determine the ergogenic impact, if any, of BCAAs [55].
Summary
Given the current evidence, the chances of BCAAs alone stimulating MPS to a physiologically-relevant extent is unlikely in humans. If BCAAs are ingested, a small rise in MPS is certainly possible, but the availability of the other EAAs will quickly become rate-limiting for sustained stimulation of MPS. Current evidence suggests that BCAA administration is not a standalone solution for promoting muscle anabolism in humans. Here are a few summary points:
- Muscle protein is made up of 20 amino acids, 9 of which must be supplied by the diet (essential amino acids; EAAs).
- The branched-chain amino acids (BCAAs) are isoleucine, leucine, and valine, and they are 3 of the 9 EAAs.
- In rodents, administration of excessive BCAA quantities may promote muscle protein synthesis (MPS).
- In humans, BCAA administration likely does not enhance MPS, and may even reduce it, resulting in a neutral, or net negative protein balance (catabolism).
- Although BCAAs stimulate enzymes that are part of the anabolic signaling cascade, this does not necessarily translate directly to muscle anabolism.
- All EAAs, including the BCAAs, are required in sufficient quantities for MPS, and anabolism, to occur
- If a diet is inadequate in any EAA, MPS cannot proceed beyond the rate at which that particular amino acid is available. This amino acid is called the rate-limiting amino acid.
- When co-ingested with other EAAs (i.e. protein), BCAAs may increase MPS if one of the three BCAAs is the rate-limiting amino acid.
- BCAA administration may reduce muscle damage if a substantial number of supplementation criteria are met (most of which are unpractical).
- In my opinion, although BCAA ingestion will do no harm, the cost of BCAA supplementation does not match up with the potential, minuscule, benefit that BCAAs provide for muscle growth (if any).
Reference
- Tipton, K.D., Gurkin, B.E., Matin, S. and Wolfe, R.R., 1999. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. The Journal of nutritional biochemistry, 10(2), pp.89-95.
- Tipton, K.D., Ferrando, A.A., Phillips, S.M., Doyle Jr, D. and Wolfe, R.R., 1999. Postexercise net protein synthesis in human muscle from orally administered amino acids. American Journal of Physiology-Endocrinology And Metabolism, 276(4), pp.E628-E634.
- Volpi, E., Kobayashi, H., Sheffield-Moore, M., Mittendorfer, B. and Wolfe, R.R., 2003. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. The American journal of clinical nutrition, 78(2), pp.250-258.
- Smith, K., Reynolds, N., Downie, S., Patel, A., and Rennie, M. J. (1998). Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am. J. Physiol. 275, E73–E78.
- Børsheim, E., Tipton, K.D., Wolf, S.E. and Wolfe, R.R., 2002. Essential amino acids and muscle protein recovery from resistance exercise. American Journal of Physiology-Endocrinology And Metabolism, 283(4), pp.E648-E657.
- Yoshizawa, F., 2012. New therapeutic strategy for amino acid medicine: notable functions of branched chain amino acids as biological regulators. Journal of pharmacological sciences, 118(2), pp.149-155.
- Layman, D.K. and Baum, J.I., 2004. Dietary protein impact on glycemic control during weight loss. The Journal of nutrition, 134(4), pp.968S-973S.
- Riazi, R., Wykes, L.J., Ball, R.O. and Pencharz, P.B., 2003. The Total Branched-Chain Amino Acid Requirement in Young Healthy Adult Men Determined by Indicator Amino Acid Oxidation by Use of l-[1-13C] Phenylalanine1. The Journal of nutrition, 133(5), pp.1383-1389.
- Shimomura, Y., Yamamoto, Y., Bajotto, G., Sato, J., Murakami, T., Shimomura, N., Kobayashi, H. and Mawatari, K., 2006. Nutraceutical effects of branched-chain amino acids on skeletal muscle. The Journal of nutrition, 136(2), pp.529S-532S.
- Wahren, J., Felig, P.H.I.P. and Hagenfeldt, L.A.R.S., 1976. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. The Journal of clinical investigation, 57(4), pp.987-999.
- Gelfand, R.A., Glickman, M.G., Jacob, R.A.L.P.H., Sherwin, R.S. and DeFronzo, R.A., 1986. Removal of infused amino acids by splanchnic and leg tissues in humans. American Journal of Physiology-Endocrinology And Metabolism, 250(4), pp.E407-E413.
- Atherton, P.J., Wilkinson, D.J. and Smith, K., 2016. Feeding modulation of amino acid utilization: role of insulin and amino acids in skeletal muscle. In The Molecular Nutrition of Amino Acids and Proteins (pp. 109-124).
- Wolfe, R.R., 2017. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality?. Journal of the International Society of Sports Nutrition, 14(1), p.30.
- Blomstrand E, Eliasson J, Karlsson HKR, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136:269S–73S.
- Kimball, S.R. and Jefferson, L.S., 2006. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. The Journal of nutrition, 136(1), pp.227S-231S.
- Crozier, S.J., Kimball, S.R., Emmert, S.W., Anthony, J.C. and Jefferson, L.S., 2005. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. The Journal of nutrition, 135(3), pp.376-382.
- Li, J.B. and Jefferson, L.S., 1978. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochimica et Biophysica Acta (BBA)-General Subjects, 544(2), pp.351-359.
- Kobayashi, H., Kato, H., Hirabayashi, Y., Murakami, H. and Suzuki, H., 2006. Modulations of muscle protein metabolism by branched-chain amino acids in normal and muscle-atrophying rats. The Journal of nutrition, 136(1), pp.234S-236S.
- Garlick, P.J. and Grant, I., 1988. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochemical journal, 254(2), p.579.
- Buse, M.G., 1981. In vivo effects of branched chain amino acids on muscle protein synthesis in fasted rats. Hormone and Metabolic Research, 13(09), pp.502-505.
- Matthews, D.E., 2005. Observations of branched-chain amino acid administration in humans. The Journal of nutrition, 135(6), pp.1580S-1584S.
- Koopman, R., Wagenmakers, A.J., Manders, R.J., Zorenc, A.H., Senden, J.M., Gorselink, M., Keizer, H.A. and van Loon, L.J., 2005. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. American Journal of Physiology-Endocrinology and Metabolism, 288(4), pp.E645-E653.
- Devries, M.C., McGlory, C., Bolster, D.R., Kamil, A., Rahn, M., Harkness, L., Baker, S.K. and Phillips, S.M., 2018. Protein leucine content is a determinant of shorter-and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. The American journal of clinical nutrition, 107(2), pp.217-226.
- Katsanos, C.S., Kobayashi, H., Sheffield-Moore, M., Aarsland, A. and Wolfe, R.R., 2006. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American Journal of Physiology-Endocrinology And Metabolism, 291(2), pp.E381-E387.
- Mitchell, W.K., Phillips, B.E., Hill, I., Greenhaff, P., Lund, J.N., Williams, J.P., Rankin, D., Wilkinson, D.J., Smith, K. and Atherton, P.J., 2017. Human skeletal muscle is refractory to the anabolic effects of leucine during the postprandial muscle-full period in older men. Clinical Science, 131(21), pp.2643-2653.
- Wilkinson, D.J., Bukhari, S.S., Phillips, B.E., Limb, M.C., Cegielski, J., Brook, M.S., Rankin, D., Mitchell, W.K., Kobayashi, H., Williams, J.P. and Lund, J., 2017. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clinical Nutrition.
- Wall, B.T., Hamer, H.M., de Lange, A., Kiskini, A., Groen, B.B., Senden, J.M., Gijsen, A.P., Verdijk, L.B. and van Loon, L.J., 2013. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clinical nutrition, 32(3), pp.412-419.
- Atherton, P.J., Kumar, V., Selby, A.L., Rankin, D., Hildebrandt, W., Phillips, B.E., Williams, J.P., Hiscock, N. and Smith, K., 2017. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clinical Nutrition, 36(3), pp.888-895.
- Koopman, R., Verdijk, L.B., Beelen, M., Gorselink, M., Kruseman, A.N., Wagenmakers, A.J., Kuipers, H. and van Loon, L.J., 2008. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. British journal of nutrition, 99(3), pp.571-580.
- Louard, R.J., Barrett, E.J. and Gelfand, R.A., 1990. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clinical science, 79(5), pp.457-466.
- Louard, R.J., Barrett, E.J. and Gelfand, R.A., 1995. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism-Clinical and Experimental, 44(4), pp.424-429.
- Nair, K.S., Schwartz, R.G. and Welle, S.T.E.P.H.E.N., 1992. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. American Journal of Physiology-Endocrinology And Metabolism, 263(5), pp.E928-E934.
- Wu, G., 2016. Dietary protein intake and human health. Food & function, 7(3), pp.1251-1265.
- Schaafsma, G., 2000. The protein digestibility–corrected amino acid score. The Journal of nutrition, 130(7), pp.1865S-1867S.
- Mathai, J.K., Liu, Y. and Stein, H.H., 2017. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). British Journal of Nutrition, 117(4), pp.490-499.
- Trumbo, P., Schlicker, S., Yates, A.A. and Poos, M., 2002. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Journal of the American Dietetic Association, 102(11), pp.1621-1630.
- Cahill Jr, G.F. and Aoki, T.T., 1971. Starvation and body nitrogen. Transactions of the American Clinical and Climatological Association, 82, p.43.
- Wolfe, R.R., 2006. The underappreciated role of muscle in health and disease–. The American journal of clinical nutrition, 84(3), pp.475-482.
- Jackman, S.R., Witard, O.C., Philp, A., Wallis, G.A., Baar, K. and Tipton, K.D., 2017. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Frontiers in physiology, 8, p.390.
- Bukhari, S.S., Phillips, B.E., Wilkinson, D.J., Limb, M.C., Rankin, D., Mitchell, W.K., Kobayashi, H., Greenhaff, P.L., Smith, K. and Atherton, P.J., 2015. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. American Journal of Physiology-Endocrinology and Metabolism, 308(12), pp.E1056-E1065.
- Greenhaff, P.L., Karagounis, L.G., Peirce, N., Simpson, E.J., Hazell, M., Layfield, R., Wackerhage, H., Smith, K., Atherton, P., Selby, A. and Rennie, M.J., 2008. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. American Journal of Physiology-Endocrinology and Metabolism, 295(3), pp.E595-E604.
- Ferrando AA, Williams BD, Stuart CA, Lane HW, Wolfe RR: Oral branched-chain amino acids decrease whole-body proteolysis. J Parenter Enteral Nutr 1995, 19: 47-54. 10.1177/014860719501900147
- Churchward-Venne, T.A., Breen, L., Di Donato, D.M., Hector, A.J., Mitchell, C.J., Moore, D.R., Stellingwerff, T., Breuille, D., Offord, E.A., Baker, S.K. and Phillips, S.M., 2013. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial–. The American journal of clinical nutrition, 99(2), pp.276-286.
- Devries, M.C., McGlory, C., Bolster, D.R., Kamil, A., Rahn, M., Harkness, L., Baker, S.K. and Phillips, S.M., 2018. Protein leucine content is a determinant of shorter-and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. The American journal of clinical nutrition, 107(2), pp.217-226.
- Katsanos, C.S., Kobayashi, H., Sheffield-Moore, M., Aarsland, A. and Wolfe, R.R., 2006. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American Journal of Physiology-Endocrinology And Metabolism, 291(2), pp.E381-E387
- Phillips, S.M., Tipton, K.D., Aarsland, A.S.L.E., Wolf, S.E. and Wolfe, R.R., 1997. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American journal of physiology-endocrinology and metabolism, 273(1), pp.E99-E107.
- Biolo, G., Maggi, S.P., Williams, B.D., Tipton, K.D. and Wolfe, R.R., 1995. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology-Endocrinology And Metabolism, 268(3), pp.E514-E520.
- Phillips, S.M., Tipton, K.D., Ferrando, A.A. and Wolfe, R.R., 1999. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. American Journal of Physiology-Endocrinology And Metabolism, 276(1), pp.E118-E124.
- Fouré, A. and Bendahan, D., 2017. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients, 9(10), p.1047.
- Rennie, M.J., Bohé, J., Smith, K., Wackerhage, H. and Greenhaff, P., 2006. Branched-chain amino acids as fuels and anabolic signals in human muscle. The Journal of nutrition, 136(1), pp.264S-268S.
- Jackman, S.R., Witard, O.C., Jeukendrup, A.E. and Tipton, K.D., 2010. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Medicine and science in sports and exercise, 42(5), pp.962-970.
- Howatson, G., Hoad, M., Goodall, S., Tallent, J., Bell, P.G. and French, D.N., 2012. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. Journal of the International Society of Sports Nutrition, 9(1), p.20.
- Greer, B.K., Woodard, J.L., White, J.P., Arguello, E.M. and Haymes, E.M., 2007. Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. International journal of sport nutrition and exercise metabolism, 17(6), pp.595-607.
- Mikulski T, Dabrowski J, Hilgier W, Ziemba A, Krzeminski K. Effects of supplementation with branched chain amino acids and ornithine aspartate on plasma ammonia and central fatigue during exercise in healthy men. Folia Neuropathol. 2015;53(4):377–86.
- Kerksick et al., 2018. ISSN exercise & sports nutrition review update: research & recommendations. Journal of the International Society of Sports Nutrition, 15(1), p.1.
Want to be the first to know when new content is created? Please enter your email address below.